Arsenic in Water
So how does the arsenic get into the water? In some cases, the arsenic in the water comes from human activities like mining and processing metals or through use of arsenic on farms. These sources were described in the “where arsenic comes from” section.
In mining, excavated rock that does not contain useful quantities of the target metals is sometimes left in a pile – it’s just rock, right? Well, this material was never exposed to air when it was buried in the earth. Once it is exposed, it can react with oxygen, with or without the help of bacteria, releasing metals and arsenic (and often acid) to the water.
In other cases, natural sources (geothermal, like the hot springs at Yellowstone National Park) contaminate surface waters.
In both of these situations, the source of arsenic is usually known and it is relatively easy to avoid drinking contaminated water.
More often the problem is with groundwater – and this is harder to detect and avoid because at the concentrations found there, arsenic can’t be seen, smelled or tasted, and there is not always an obvious source.
Surface Water – Groundwater: What’s the difference?
To understand why arsenic gets in to groundwater, you need to understand where it comes from. That means knowing a little about the water cycle (or hydrologic cycle). This will also help you understand why this is usually a groundwater problem, and rarely affects surface water. We’ll start with a description of two kinds of water: surface water and groundwater.
Surface water is the water in the oceans, streams, lakes and rivers. For most human uses, ocean water is too salty to be used, so we will consider it the source of most of the water that enters the hydrologic cycle and leave it as that. Even though the plants and animals that live in the ocean would probably object!
The picture below shows how the hydrologic cycle works. It starts in the oceans, where energy from the sun causes water to evaporate. In the atmosphere it condenses in the form of clouds. The clouds move with prevailing winds until the drop sizes get too big and the drops are too heavy to stay in the atmosphere, causing rain. If the cloud is over the ocean when that happens, the water will just cycle again. If it is over land, it will contribute to the land-based hydrologic cycle as seen in the picture. The rain (or snow) water runs over the surface into rivers and lakes and eventually back to the ocean, or it travels thorough the soil to the groundwater and flows underground. Again, it ultimately ends up back in the ocean.
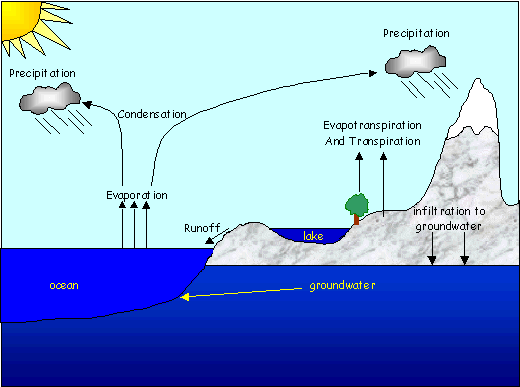
Surface waters are found at low points in “watersheds”, which are land areas that drain toward the surface water body. Any snow that melts or rain that falls in the catchment area that doesn’t soak in to the ground can run downhill over the surface to the stream or lake at the low point. Surface water bodies can also be fed by groundwater. This water flows, always downhill, toward the sea.
The speed of the water flow depends on how much water is in the system and how big the water body is. If a lot of water is traveling through a small channel, like a stream in the spring, when a lot of snow melts all at once, it will have to travel very fast. If the same amount of water is flowing through a huge lake basin, it will take a much longer time for it to travel the same distance. Some lakes have “residence times” that are measured in years. The residence time is how long something spends in a particular place. In general surface water moves a lot faster than the water that travels underground.
The table shows typical residence times of water molecules in different water bodies. You can see that water travels through rivers much faster than lakes or the soil beneath the surface!
| Location | Typical Residence Time |
|---|---|
| Atmosphere | 9 days |
| Rivers | 2 weeks |
| Large Lakes | 10 years |
| Shallow Groundwater | 10s-100s years |
| Top Layers of the Ocean | 120 years |
| Whole Ocean | 3000 years |
| Deep Groundwater | Up to 10,000 years |
| Antarctic Ice Cap | 10,000 years |
Since surface water travels over the ground, it can pick up and carry along any chemical, particle or organism that is small enough to be swept along in the flow or that can be dissolved in water. That means that bits of soil, sticks, soluble chemicals and microorganisms can all be carried from the land into surface waters. Soil that’s not held in place by plant roots, bacteria, manure or pesticides that are spread on fields, road dust, oil from cars, paint thinner dumped on the ground – all of these things can be swept by rain water into the rivers and lakes. Since surface waters can contain so many “impurities”, they generally need to be treated before it is safe to drink. Typically they are filtered to remove the solids and disinfected to kill any bacteria or other microorganisms that can cause disease.
Groundwater is the underground water that we tap when we dig a well for drinking water. If it rains or you pour water on the ground, the water travels downward because of gravity. It will trickle down through the spaces between soil or sand grains in the surface soil and keep going (if it is not sucked up by plants or evaporated) through the unsaturated or “vadose” zone until it reaches the water table. That’s the top of the “saturated zone”: the place where all of the spaces between the sand grains are filled with water. It’s called the saturated zone because the soil spaces are “saturated” with water. Right on top of the saturated zone there is a zone called the capillary fringe. Soil acts like a sponge to suck up water, so this zone has more water than the vadose zone on top of it, but it is not completely saturated with water.
The water is stopped from continuing down to the center of the earth by a layer of rock or clay that it can’t travel through. The column of water on top of the impermeable layer travels slowly downhill toward a low point. It may be released as a spring, seep into a lake or river, travel to the ocean underground or get pumped up from a well to somebody’s kitchen sink.
While the water moves toward the water table, the soil acts like a huge filter, leaving the particles and the microorganisms in the upper layers of the soil. This purifies the water and reduces the risk of being exposed to disease-causing organisms. But there is a trade-off. Since the water moves so slowly through the spaces between sand and gravel and through the cracks in the bedrock, it is in contact with the minerals of the subsurface for a long time. This allows the water to slowly dissolve the minerals in the soil.
So while groundwaters are usually very low in suspended solids and microorganisms, they tend to have higher concentrations of dissolved metals and other salts. Groundwater with a lot of dissolved salt is sometimes described as “hard water”, when the concentrations are high enough to precipitate in pipes and hot water heaters. To prevent clogging of pipes and other problems like the rust-coloured stains you can get on your white clothes if water is high in iron, groundwater is often treated to remove the salts. This can be done by forcing the salts or metals to precipitate out of solution, then filtering out the solids; or by passing the water through a column that traps the metals. This will be discussed in the Treatment options section.
Ok, so back to arsenic. When the source of arsenic is the bedrock and soil material, surface water doesn’t really stay in contact with the source of the arsenic for long enough to dissolve much of it, and the concentration stays low. The exceptions to this rule would be if an industry that uses arsenic releases wastewater to a river, or if a hot spring drains into a stream.
On the other hand, groundwater stays in contact with bedrock or soil material for decades or even centuries, allowing it to accumulate a much higher concentration of arsenic, if it’s present in the rock. So that’s why arsenic is usually higher in groundwater than surface water.
The thing is, not all groundwater sources are high in arsenic. Even some groundwaters that come from areas with a lot of arsenic in the rocks are low in arsenic, so there are other factors that affect the concentration. The only way to be sure is to test the water.
It is difficult to get a clear estimate of how many people are exposed to high arsenic, because not all drinking water is tested for arsenic. Public water supplies in the United States all have to test the water for arsenic and keep it below a maximum of 10 mg/L (that’s parts per billion – or something like 10 seconds in 32 years). This was recently reduced from 50 mg/L to protect people from the chronic (long term, small dose) health effects of arsenic. So community water supplies are all tested regularly.
The big problem is where people have their own wells. There is no requirement to test for arsenic, so many people may be exposed and not know it (remember, you can’t see, smell or taste arsenic). It would help a great deal if more was known about the factors that contribute to high arsenic levels in groundwater, so that high risk areas can be found, and people living there can be advised to test their wells.
If you get your drinking water from a well, the safest thing is to test your water regularly (once every 3-5 years – you might want to check at different times of year). If the concentration is above 10 ppb, you should think about drinking bottled water, or treating your drinking and cooking water.
What difference does it make if arsenic gets into groundwater?
- About 50% of drinking water supplies in the USA use groundwater sources
- About 60% of people in Maine drink water from groundwater sources, and in rural areas about 90% do!
If you would like to learn more about groundwater quality and contamination, the EPA has an excellent Groundwater Primer that you should explore!
