New research significantly advances production of polyrotaxanes
Researchers have made a significant advance in the synthesis of high-energy mechanically interlocked polymers (MIP) used in exotic materials, according to a new study in the prestigious journal Science.
University of Maine professor R. Dean Astumian, a theorist in the Department of Physics and Astronomy, is a corresponding author of the study, along with Sir James Fraser Stoddart, 2016 Nobel Laureate in Chemistry and Board of Trustees Professor of Chemistry at Northwestern University, and Xiaopeng Li, an expert in mass spectrometry at the University of South Florida. The article, “A precise polyrotaxane synthesizer,” describes the work, led by postdoctoral researcher Yunyan Qiu at Northwestern University, which demonstrates how artificial molecular machines (AMMs) — that is, artificially synthesized molecular components that produce machine-like movement — can be harnessed to produce polymers such as polyrotaxanes with incredible precision previously unachievable through other methods.
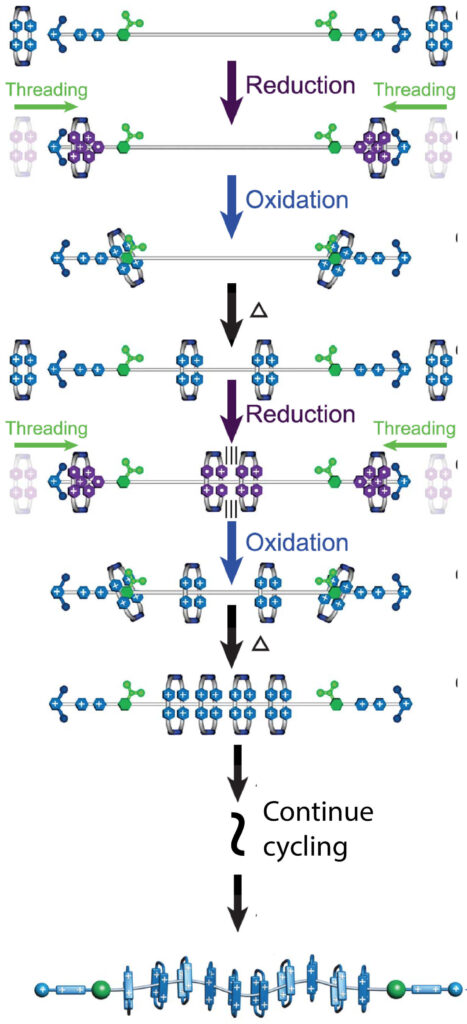
The authors report the assembly line-like emergence of polyrotaxanes with increasingly larger numbers of rings and with higher energies by harnessing artificial molecular pumps to deliver rings in pairs onto a polymer chain by cyclical oxidation-reduction of the components. This programmable strategy offers improvements over existing, template-driven methods for polymer synthesis. Templation uses self-assembly and molecular recognition in the synthesis but does not provide control over their final properties. The newly reported use of artificial molecular pumps, by contrast, leads to the precise incorporation of two, four, six, eight, and 10 rings carrying 8+, 16+, 24+, 32+, and 40+ charges, respectively, onto polymer dumbbells (DB+6).
The palindromic arrangement of “pumping cassettes” on either end of the DB molecule separates the long polymer chain from the bulk solution. Because of the kinetic asymmetry arising from the electrostatic barrier facing the solution and a steric barrier facing the polymer “collecting chain,” rings are successively pumped onto the site between the barriers when in the reduced state (shown in purple) and expelled to the collecting chain when in the oxidized state (shown in blue) in a mechanism known as an energy ratchet. Up to ten rings were pumped onto the collecting chain to form a very high energy and highly charged molecule. Creation of this molecule would have been impossible using standard synthetic techniques.
Producing these mechanically interlocked polymers with a precise number of rings is also important because it makes it possible to find out how their properties change as a function of the number of rings. The method allows scientists to fine-tune the properties of these polymers by adding a prescribed number of rings onto the polymer (dumbbell) chain.
The research has important implications for slide-ring gels, battery electrode materials, drug delivery systems, and other technological applications. Astumian points out that the research is also important for understanding one of the deepest questions in chemistry: “What are the principles by which simple matter becomes complex? A key point is that while thermodynamics determines the most likely structures near equilibrium, kinetics plays the dominant role in selecting structures when far from equilibrium.”
Stoddart, who supervises the lab at Northwestern where Qui conducted the experiments, agrees: “The work tells us that we are drawing ever closer to producing a wholly synthetic machine that is operating similarly to ion pumps that regulate the electrical potential across biological membranes in our cells.” Though he cautions that we are not there yet, this work might form the basis for a prototype of, for example, an all-organic battery.
Contact: Brian Jansen, brian.jansen@maine.edu
