Harnessing Environmental RNA to Understand Blue Mussel Life Stages
By Evan Bartow, Writing Intern

Assisting in the development of new environmental genomics research, Dave Ernst (postdoctoral scientist at Bigelow Laboratory for Ocean Sciences) works to develop the research tools that leverage environmental RNA (eRNA) as part of the NSF EPSCoR RII Track-1 Maine-eDNA grant. The use of eRNA detection tools can help expand the current abilities of environmental DNA (eDNA) researchers, increase the information available for larval ecology, and provide additional benefits to mussel farmers.
Recently demonstrated by Ernst, eRNA is another way that geneticists are able to monitor the distribution of populations and species throughout a marine ecosystem. DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) are both parts of the genetic code that is within all living organisms.These molecules differ in their structure, function, length, and nucleotides. Further differentiating the two, DNA is always present in the cell whereas RNA must be transcribed or expressed during protein synthesis. In an environmental context, eDNA can only detect at the species or population level which on its own is powerful. However eRNA can provide finer spatiotemporal resolution and, because it is dependent on gene expression, identify finer details that eDNA cannot like life stages or even potentially physiological states. Researchers worried that eRNA degraded too quickly in water compared to eDNA but this seeming downside is what Ernst argues makes it a powerful tool. This short degradation time means that eRNA cannot travel that far from its source providing that increased spatiotemporal resolution. Additionally and unlike eDNA, RNA is only produced by living organisms. While these tools have different applications, both eDNA and eRNA are tools scientists can use to identify the distribution and location of various species.
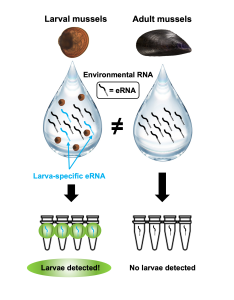 The development of these eRNA detection tools was driven by the desire to detect and quantify commercially significant shellfish species. Ernst explained the “goal of the project was to specifically detect the larval stages of blue mussels.” Across the state of Maine, mussel farmers have experienced seed recruitment failures which has limited their ability to culture mussels for harvest. To help improve recruitment, Ernst is developing the methods needed to monitor mussel larvae within the water column. Mussel seed recruitment is difficult due to the small size of mussel larvae and the challenging nature of collecting the species. In Maine, mussel farmers typically use suspended culture where they hang ropes into the water that swimming mussel larvae settle and grow. Given their size and morphological similarities with other local bivalves, it is difficult to determine when and where mussel larvae are and the best time and location for larvae collection. Through the use of eRNA detection, researchers and farmers can use a simple water sample to pinpoint the exact life stages of these mussels and better understand their location throughout the water column.
The development of these eRNA detection tools was driven by the desire to detect and quantify commercially significant shellfish species. Ernst explained the “goal of the project was to specifically detect the larval stages of blue mussels.” Across the state of Maine, mussel farmers have experienced seed recruitment failures which has limited their ability to culture mussels for harvest. To help improve recruitment, Ernst is developing the methods needed to monitor mussel larvae within the water column. Mussel seed recruitment is difficult due to the small size of mussel larvae and the challenging nature of collecting the species. In Maine, mussel farmers typically use suspended culture where they hang ropes into the water that swimming mussel larvae settle and grow. Given their size and morphological similarities with other local bivalves, it is difficult to determine when and where mussel larvae are and the best time and location for larvae collection. Through the use of eRNA detection, researchers and farmers can use a simple water sample to pinpoint the exact life stages of these mussels and better understand their location throughout the water column.
In collaboration with the Downeast Institute, Ernst examined RNA that was extracted from mussels at several different life stages. Ernst used this previously sequenced RNA to help him look for differences in gene expression patterns across mussel development. To build on this, he plans to examine and sequence additional RNA data that he collected. In his initial work, Ernst was able to find larvae-specific gene patterns that were used in the development of larval detection assays.These tools have the ability to specifically target larval biomarkers which identify the exact stage of the target blue mussels, proving what many believed improbable to be possible.
This success has provided Ernst and other researchers with opportunities for future research and development. For mussel farmers, Ernst plans to “incorporate these assays into toolkits that mussel farmers can use on their lease.” This would help farmers test the waters for mussel larvae and help mitigate future recruitment failures. Ernst believes that the “combination of eRNA and eDNA tools can be very powerful for aquaculture in Maine to promote the sustainability of the industry”
The ability to detect life stages using eRNA enables further study of larval ecology. “Marine larval ecology is still very much a black box,” Ernst explained. While researchers can collect information regarding larval ecology, relatively little is known about the behavior and dispersal of these early developmental stages, and how they are affected over time. Due to eRNA’s large array of applications, the development of eRNA research tools will support Maine mussel farmers, provide new information to the study of life cycle dynamics, and expand the capabilities of environmental genomic researchers.
