NMR Facility Goals and Information
 The University of Maine Chemistry Department’s goal is to ensure the NMR Facility provides researchers with optimum NMR tools and resources. Our NMRs serve not only the Chemistry Department but also researchers from other research facilities. We encourage researchers from colleges and businesses across Maine to assess whether NMR can help them to achieve their goals. As part of this initiative, this web page provides an introduction to NMR. It also provides information and news for potential NMR users on the status of the Department’s NMRs and on how they are being utilized in various research projects.
The University of Maine Chemistry Department’s goal is to ensure the NMR Facility provides researchers with optimum NMR tools and resources. Our NMRs serve not only the Chemistry Department but also researchers from other research facilities. We encourage researchers from colleges and businesses across Maine to assess whether NMR can help them to achieve their goals. As part of this initiative, this web page provides an introduction to NMR. It also provides information and news for potential NMR users on the status of the Department’s NMRs and on how they are being utilized in various research projects.
Contents:
What can NMR do for you?
How does it work?
Is NMR difficult to use?
How to start utilizing NMR
University of Maine NMR Facility Administration
More Information about Learning NMR
Examples of how NMR is used at the University of Maine
What can NMR do for you?
 NMR is used to easily identify unknown chemical compounds without the need of a chemistry lab. For simple compounds the process usually takes about ten minutes. NMR is used to identify the chemicals found in our environment like the pesticides found in our gardens, hydrocarbons found in our fuels and in the air we breathe, acids found in the foods we eat, and even in the proteins and DNA that life is based on. The list is endless. As one of the most powerful tools in chemistry for identifying chemicals, NMR has vast potential in areas like biology, food science, chemical engineering and marine science.
NMR is used to easily identify unknown chemical compounds without the need of a chemistry lab. For simple compounds the process usually takes about ten minutes. NMR is used to identify the chemicals found in our environment like the pesticides found in our gardens, hydrocarbons found in our fuels and in the air we breathe, acids found in the foods we eat, and even in the proteins and DNA that life is based on. The list is endless. As one of the most powerful tools in chemistry for identifying chemicals, NMR has vast potential in areas like biology, food science, chemical engineering and marine science.
How does it work?
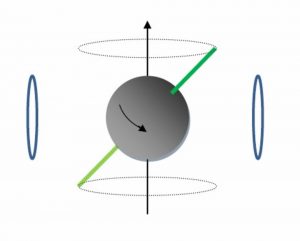
It is an amazing fact that most atomic nuclei have a quantum spin, a fixed mass, and behave like tiny magnets. When placed in a strong magnetic field they can precess such that they draw out the dotted circles as shown here. The spinning wheel in this video demonstrates this motion. The precessing frequency can be measured accurately by the wire coils shown here in blue. A driving alternating current placed on the same coils can be used to make the nuclei precess. Most elements in the periodic table have a unique precession frequency when placed in a fixed magnetic field. By measuring the unique frequencies found in a sample, we can determine the type of chemical elements contained in that sample. In our 400 MHz magnet, hydrogen nuclei precess at 400 million cycles per second, carbon at 100 MHz and nitrogen at 29 MHz. The frequency of this precession can be determined from the oscillating current in the wire coils as shown here in this simulation.
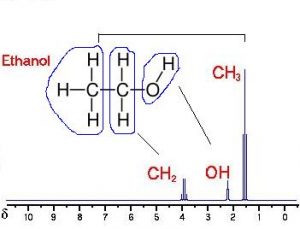
But NMR is even more powerful than that. The frequency also depends on other nuclei that are chemically bonded to the nuclei we are observing. The bonds actually shift the precession frequencies slightly. With this frequency shift information, a trained chemist, or someone using smart software, can identify not just the atomic elements in a chemical sample, but also the compounds made up of those elements in a sample. For example, the hydrogen NMR spectrum shown here of intensity versus ppm of frequency shift, reveals that a compound in a sample has CH3, CH2 and OH peaks. A trained chemist or someone using chemical analysis software could easily identify this compound as ethanol.
Is NMR difficult to use?
NMR technology has evolved over time and has become easier to use. In many cases, a user can insert their sample, press a button on a computer screen and within a few minutes collect all the data they’ll need to identify their sample. They can then transfer that data into an NMR analysis program installed on their computer that includes a chemical library of spectra, and use it to identify the chemical compounds in their sample. Here is a typical sequence for NMR users at the University of Maine.
- Reserve a slot of NMR time using our online NMR scheduler. Users can reserve NMR time remotely, from a few minutes to hours, from their computer, tablet or smart phone, or locally in the NMR room by going to the site http://chemistry.um.maine.edu/.
- The sample is then placed in the NMR. Samples are dissolved in a special deuterated solvent, like deuterated water or chloroform and placed in a glass NMR tube.
- Check something called a lock signal, press two buttons and wait, then transfer the data to a computer with data analysis software and identify the compound. The entire data collection process takes a few minutes.
- Don’t forget to sign out and let other users know online that the NMR is free.




NMR has evolved as a powerful tool for identifying increasingly complex molecules, like proteins and DNA. Identifying compounds like these may require much longer data collection times and more NMR expertise.
How to Start Utilizing NMR
Researchers begin by sending their graduate and undergraduate students to the NMR Facility Manager, David Labrecque, 207-949-3982, for NMR training. Students are often paired with other trained users as part of their training. New users are trained to do basic NMR in about 3 to 5 hours. This includes a one hour lecture, an hour of exercises and a worksheet that must be completed satisfactorily. New users start out with a “learner’s permit” where they are allowed to use the NMRs weekdays, between 8 and 4, when other trained users are around to answer questions and provide general assistance.
We’ve developed NMR training software to help new users learn about NMR and how to use it safely. Designed as an informative and interactive game, most beginners actually enjoy learning about using NMR without the stress of worrying about damaging a real instrument. After they complete this session, trainees get to do some NMR analysis with a decommissioned NMR that works the same as the bigger working NMRs. After completing a worksheet successfully, novice users get a learner’s permit to use the University’s 400 MHz NMRs, where they can collect and analyze real data from real samples. After a quick final check, they can use the NMR anytime. Another training session is required to use our new 500 MHz Bruker Neo NMR.
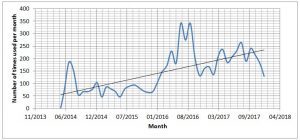 An increasing number of users have been taking advantage of the University’s NMR facility. This graph of NMR user sessions documents a 47% increase in use since June 2014.
An increasing number of users have been taking advantage of the University’s NMR facility. This graph of NMR user sessions documents a 47% increase in use since June 2014.
We encourage faculty at the University of Maine and at other colleges, and researchers at hospitals, Jackson lab, and those in the business community with a need for NMR analysis to assess how NMR can aid their endeavors, and if warranted, to attend our yearly NMR workshop, which includes lectures and hands-on instrument time.
University of Maine NMR Facility Administration

The committee oversees an NMR Facility Manager and an NMR Advanced Applications Consultant. The facility manager maintains the NMR facility and provides NMR workshops and one-on-one instruction on an as-needed basis. The advanced applications consultant provides advanced training, collaborates with researchers and offers NMR research services to the on- and off-campus research community. The University of Maine Chemistry Department also offers various courses that include NMR components, and offers a yearly NMR seminar open to researchers and those in the business community with a potential need for NMR.
 The Chemistry Department has a 400 MHz Agilent/Varian Inova NMR running VnmrJ 4.3 software 6.1 C, and a new 500 MHz Bruker Neo NMR running the latest Topspin software. The Inova has several probes including two gradient 4-nuke broadband types, a gradient indirect detection and a solid-state MAS probe. The Department also has a 45 MHz picospin desktop NMR with capillary input. Our NMRs can be run remotely from anywhere in the world using Zoom software. We use this for remote training and outreach programs.
The Chemistry Department has a 400 MHz Agilent/Varian Inova NMR running VnmrJ 4.3 software 6.1 C, and a new 500 MHz Bruker Neo NMR running the latest Topspin software. The Inova has several probes including two gradient 4-nuke broadband types, a gradient indirect detection and a solid-state MAS probe. The Department also has a 45 MHz picospin desktop NMR with capillary input. Our NMRs can be run remotely from anywhere in the world using Zoom software. We use this for remote training and outreach programs.
More Information about Learning NMR
NMR stands for Nuclear Magnetic Resonance. The word “nuclear” refers to the nucleus of common stable atoms and has nothing to do with radioactive compounds or with radioactivity in general.
Those who are new to NMR might want to check out: https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance_spectroscopy
For those who want more depth, check out this site beginning with Chapter 3: https://www.cis.rit.edu/htbooks/nmr/inside.htm
Contact David Labrecque, 207-581-1195, DavidLab@Maine.edu, for more information.
Examples of how NMR is used at the University of Maine
Characterization of Glycans Produced by Automated Synthesis
Matthew Brichacek, Assistant Professor, Department of Chemistry, University of Maine
Specific Aims: Structurally Characterize Synthetic Glycans Produced by Automated Synthesis
(Glycans are typically attached to proteins and on the exterior surface of organic cells.)
NMR Metabolomics of Blue Mussels
Dr. Karl Bishop, Husson University
Collaborator: Dr. Paul Rawson, School of Marine Sciences, University of Maine
Specific Aims: Use 1D and 2D NMR spectroscopy to identify and quantitate metabolites in Mytilus edulis (blue mussels) and to correlate changes in these metabolites in response to environmental changes and stressors.
Mechanistic investigations of metal mediated thiolate-disulfide exchange reactions
Alice Bruce, Professor and Chair, Department of Chemistry, University of Maine and Mitchell Bruce, Professor, Department of Chemistry, University of Maine
Specific Aims: Characterize reaction products and measure initial rates for reactions of gold and zinc thiolate complexes with glutathione disulfide and other biologically relevant disulfides in aqueous solution. (Disulfide bonds are in a wide range of biological molecules.)
High Resolution NMR MicroProbes for Bioanalysis
Scott Collins, Professor, Department of Chemistry; the Graduate School for Biomedical Sciences & Engineering, University of Maine
Specific Aims: Generate an NMR meander microcoil probe to measure the bonding structure of chromatin by fluidic shear stress. (Chromatin is basically the DNA, protein and RNA found in cells.)
Biofuels and Heterogeneous Catalysis
Brian Frederick, Associate Professor, Department of Chemistry; Member, Laboratory of Surface Science and Technology (LASST) and Forest Bioproducts Research Institute (FBRI), University of Maine
Specific Aims: Identify composition of biofuels and reaction mechanisms in heterogeneous catalysis
Sustainable polyesters with selectable, lignin derived pendent groups
William M. Gramlich, Associate Professor, Department of Chemistry, University of Maine
Specific Aims: Synthesize lactone monomers with different lignin derived pendent groups and characterize the pendent group effect on polymerization mechanism
Conversion of Biomass to Chemicals and Fuels
Thomas Schwartz, Assistant Professor, Chemical and Biological Engineering, University of Maine
Specific Aims: Definitively identify intermediates, side products, and impurities in biomass feedstocks and biobased chemicals
