Students use UMaine Hess Lab to research universal treatment for flu virus
Students at the University of Maine are using physics to fight the flu. A group of undergraduate and graduate students, led by physics professor Sam Hess, is doing single molecule microscopy combined with molecular simulations to learn about how influenza viruses mutate and hijack the cells of their host.
Their discoveries could lead to the creation of a universal flu treatment with potential applications for other viruses, offering more effective treatment options to defend against cold and flu season. Scientists model seasonal influenza vaccines after a strain of the virus they believe will be the most prominent that year. If a mutated strain circulates that is different from the model, the vaccine doesn’t protect fully against the virus.
“Sometimes scientists get it wrong, and the strain that’s actually popular that year is not one of the ones that the vaccine targeted, or it’s not a great match,” Hess said. “If you’re not that well protected by the vaccine, we need ways to fight whatever virus might be coming around.”
Hess and his students use super resolution microscopy and fluorescence spectroscopy — methods that illuminate the smallest structures in cells with lasers — to capture images of molecular interactions that are used together with simulations to create a model of what is happening at the nanoscale.
The lab goes dark when the microscope is ready to use; overhead lights would ruin the image by prematurely activating the sample. Specks of dust floating through the path of lasers are all that’s visible outside the small view in the microscope lens.
“Everybody gets excited when they see the individual molecules flash in front of their eyes,” Hess said. “It’s like sparkles on a moonlit lake or twinkling stars or fireflies. It’s not super bright, but you can see those flashes with your own eyes.”
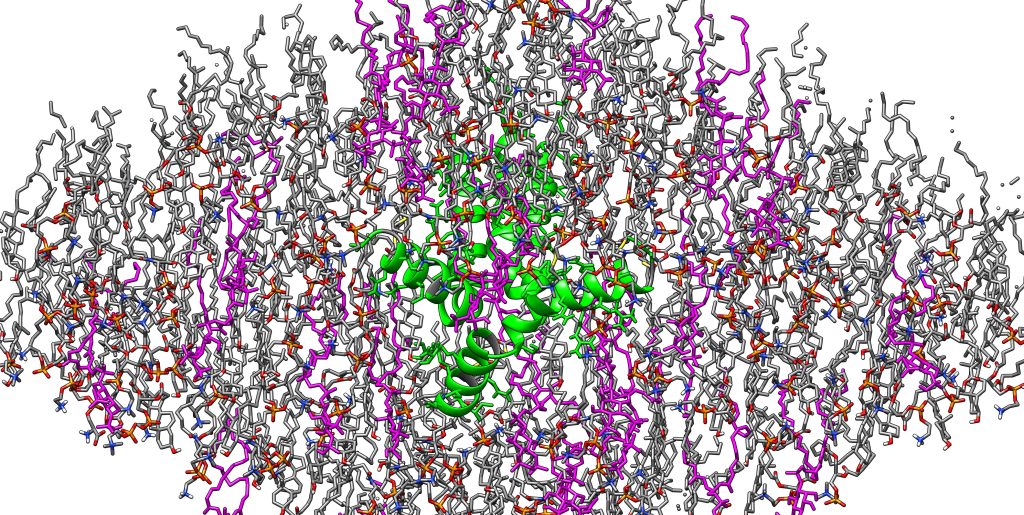
Images capture the interaction between a spike protein on the surface of the flu virus — hemagglutinin (HA) — and the outermost layer of the cell, the plasma membrane.
When a virus attacks a cell, it attaches to the cell membrane via HA and becomes an enclosed compartment surrounded by the cell membrane. Over time, the virus infects the cell by opening a hole in the cell membrane and sending in viral RNA. Once the cell is infected, it produces more of the HA and other viral proteins, which assemble into new viruses and can attack and infect more cells.
Once more HA is produced by an infected cell, a tail on HA binds with phosphatidylinositol phosphates (PIPs) — host cell membrane lipids that regulate cell trafficking, signaling and other processes — and uses these PIPs to assemble new viruses. This is the basis of national research aimed at understanding how spike proteins interact with the host cell to achieve successful infection.
UMaine students are narrowing their research on how the tail of HA interacts with PIPs and whether that interaction can be the foundation of universal flu treatment, such as antiviral therapy or antibody treatments.
“This little tail has features that are always there,” Hess said. “In a virus that mutates, what’s conserved must be conserved for a reason, or it would just mutate like the other parts, right?”
Current vaccines are developed to attack the HA ectodomains, protrusions that are projected from the viral membrane and highly susceptible to mutation, as opposed to the small tail of HA that remains the same over time.
NIH grant supports student research
For the next three years, a $430,000 grant from the National Institutes of Health is supporting the Hess Lab and the success of undergraduate and graduate students who research there. Hess said the essence of the grant is to study PI4P, one of three phosphates found in the cell membrane.
The phosphate PIP2 is known to play a role in infections caused by several different viruses, but treatments that only target PIP2 don’t completely stop the virus. Hess and his students are researching to what extent PI4P is involved in infecting the cell, and whether PI4P and PIP2 are working together.
By learning how to work with super resolution microscopy and fluorescence spectroscopy, students are also gaining experience that will help them stand out to employers.
Physics students such as undergraduate Tiance Yang will be able to leverage their experience from the Hess Lab to pursue a career in biophysics, a science that applies principles of physics to understanding biological systems.
Yang, whose focus is on molecular dynamics — a key study area of biophysics — will use his research in the lab to complete his senior capstone project. He runs simulations on how the spike protein in a different viral respiratory illness, with similar molecular structure to influenza, interacts with PIPs, then analyzes the results with charts and graphics.
“I would like to go to grad school,” Yang said. “This knowledge is helpful, because if I want to ever get into the more biomedical side, it gives me an advantage.”
Graduate student David Winski has worked in the Hess Lab for five-and-a-half years and is preparing to defend his thesis and graduate in the spring. He then plans to work professionally in biophysical research. He has worked with Hess to perform mutations of the HA binding tail, image it with super resolution microscopy and then simulate it at the atomic level by applying Newton’s Laws of Motion.
Winski also teaches undergraduate students — many of whom are physics majors — basic biology and how it applies to their physics research. In-lab biology is focused on cell processes and what’s happening within the human body, while the work in physics allows students to visualize what’s happening by combining results from experiment and simulation into a molecular model.
“We’re trying to make this more broad than just applying it to influenza and the (HA) spike,” Winski said. “Can we apply what we’ve been finding out with influenza, apply that to other viruses in the world? Can we somehow make this even more broad and come up with potentially, ideally a universal vaccine?”
Contact: Ashley Yates; ashley.depew@maine.edu