New paper on the effects of feedback phosphorylation of the RGS, Sst2.
We have recently published a paper in Life Science Alliance, entitled “Phosphorylation of RGS regulates MAP kinase localization and promotes completion of cytokinesis.” This work was spearheaded by master’s student Will Simke and PhD student Cory Johnson, co-first authors on the final work.
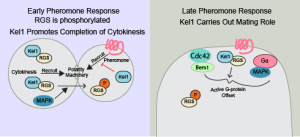
When yeast respond to mating pheromone, they must complete their current round of mitosis and cytokinesis, arrest in G1, and then polarize towards the active pheromone receptor. While the mechanism of G1 arrest is fairly well understood, the mechanisms by which the cell stops the active pheromone receptor from co-opting the polarity machinery before the yeast have finished cytokinesis is not understood. We are interested in desensitization of the pheromone pathway by the Regulator of G-protein Signaling (RGS), Sst2. The RGS serves as a GTPase Activating Protein for the large G-protein, Gpa1, at the top of the pheromone pathway. RGS binding induces the G-protein to turn off, but there are many aspects of RGS signaling to the G-protein that are not understood. It has long been known that the RGS is phosphorylated by MAP Kinase during the pheromone response, but there was no clear physiological outcome from this modification.
We have found that the RGS, Sst2, is phosphorylated during the pheromone response to regulate its interaction with the kelch-repeat protein, Kel1. Kel1 regulates actin polymerization by formins, and also plays a role in the Mitotic Exit Network (MEN). The feedback phosphorylation of the RGS by MAP Kinase during the pheromone response helps the cells prioritize the completion of cytokinesis prior to repurposing the cell polarity machinery for pheromone receptor-mediated polarized growth. At later times in the pheromone response, phosphorylation of the RGS alters where MAP Kinase is active relative to the polar cap.