Metal-Sulfur Chemistry
We became interested in metal-sulfur chemistry more than a decade ago as a result of our interest in gold(I) metallodrugs which are used to treat rheumatoid arthritis.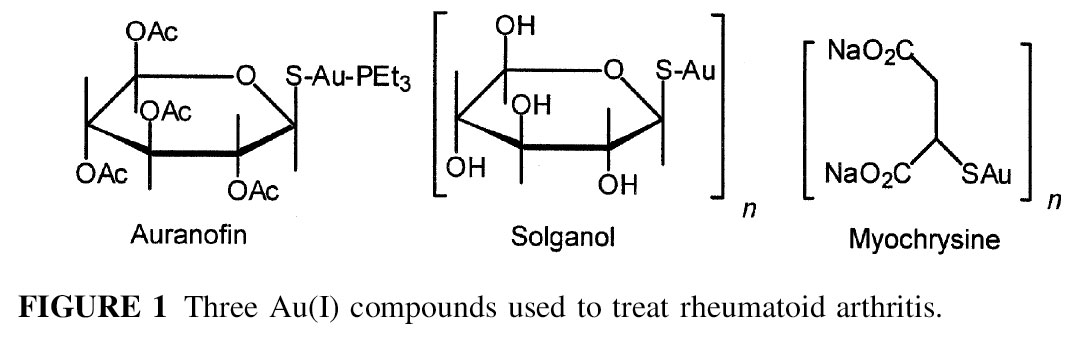
No one knew then how these metallodrugs worked and this is still true today. The notion of gold atoms running around in cells doing anything useful may be a silly image.
But, as it turns out, gold drugs effectively diminish the debilitating effects of rheumatoid arthritis in some patients. Heavy metals, such as gold, are usually considered as poisons, and there is some truth to that since some patients experience unpleasant side effects while taking gold medicines. As chemists, we have been interested in gaining a better understanding of the intracellular reactions of gold in order to bettter understand the pharmacological effects. This should in turn lead to development of better medicines, with fewer side effects.
There are no naturally occurring gold containing enzymes and the prevailing assumption is that in healthy cells metals are tightly sequestered, although this notion has been challenged a bit recently. Since metallothioneins are molecules that are known to accumulate zinc and gold through bonding to cysteine sulfurs, we are considering the intriguing possibility that these metal-thiolates may serve as a source of metals to influence cellular chemistry. Glutathione is a sulfur-containing tripeptide that is in high concentration (mM) in cells. Under oxidative stress, the concentration of glutathione disulfide, which is a marker for rheumatoid arthritis, rapidly increases.  We have been investigating the reactions of zinc-thiolates and gold-thiolates with disulfides and have been fascinated with learning how these reactions occur. A comparison of metal-assisted sulfur reactions and the ubiquitous thiol-disulfide reaction is especially intriguing, since thiol-disulfide exchange is critical to many processes in cellular regulation. Our results are helping us think about how gold may mimic or compete with zinc and thereby influence cellular chemistry. Ultimately our research is aimed at understanding molecular mechanisms of metal dependent life processes and thus it is an example of research in the emerging area of metallomics. We are writing up several papers now that describe our recent results. Check back here for updates. As we build this site, we will summarize our research findings over the years and recent results as papers are published.
We have been investigating the reactions of zinc-thiolates and gold-thiolates with disulfides and have been fascinated with learning how these reactions occur. A comparison of metal-assisted sulfur reactions and the ubiquitous thiol-disulfide reaction is especially intriguing, since thiol-disulfide exchange is critical to many processes in cellular regulation. Our results are helping us think about how gold may mimic or compete with zinc and thereby influence cellular chemistry. Ultimately our research is aimed at understanding molecular mechanisms of metal dependent life processes and thus it is an example of research in the emerging area of metallomics. We are writing up several papers now that describe our recent results. Check back here for updates. As we build this site, we will summarize our research findings over the years and recent results as papers are published.
Gold has a strong relativistic effect. The propensity for bonding interactions between closed shell d-10 gold(I) atoms was noted in both theoretical and experimental studies. These interactions, coined aurophilicity, were known to be weak. We had made a series of gold(I) complexes, most were white, but one was bright yellow! We thought that aurophilic effects may be responsible for the yellow color. Click the Aurophilicity link above for more information about our research in this area.
Narayanaswamy, R.; Young M. A.; Parkhurst, E.; Ouellette, M.; Kerr, M. E.; Ho, D.; Elder, R. C.; Bruce, A. E.; Bruce, M. R. M. Inorg. Chem., 1993, 32, 2506-2517.
Jones, W.; Yuan, J.; Narayanaswamy, R.; Young, M.; Elder, R.; Bruce, A.; Bruce, M.Inorg. Chem. 1995, 34, 1996.
Sulfur-Based Electrochemistry with Gold(I) Complexes
When we started our electrochemical investigation, the literature was unclear on if gold(I), or sulfur, or phosphorus based oxidation would occurred in complexes like those shown in Figure 1. Since oxidation of gold(I) could lead to gold(III), a toxic form of gold, it was important to understand the redox chemistry of gold(I) complexes. What we found surprised us. Click the Sulfur-Based Electrochemistry link above for more information about our research in this area.
Chen, J.; Jiang, T.; Wei, G.; Mohamed, A.A.; Homrighausen, C.; Krause Bauer, J.A.; Bruce, A.E.; Bruce, M.R.M. JACS, 1999, 121, 9225.
Mohamed, A.A.; Bruce, A.E.; Bruce, M.R.M. “Metal-Based Drugs, 1999, 6, 233.
Mohamed, A.A.; Bruce, A.E.; Bruce, M.R.M. Organic Derivatives of Silver and Gold, Patai, S. and Rappaport, Z., Eds., John Wiley & Sons, England, 1999.
Jiang, T.; Wei, G.; Turmel, C.; Bruce, A.E.; Bruce, M.R.M. Metal Based Drugs, 1994, 1, 419.
Although a gold(I)-gold(I) interaction is weak, it can promote unusual chemistry. For example, the formation of a gold(I)-gold(I) interaction that makes the molecule photochemically reactive. This and stories about the gold(I)-sulfur nanocluster and gold liquid crystal compounds shown below are found at: Unusual Gold(I) Chemistry.
Chen, J.; Mohamed, A. A.; Abdou, H. A.; Krause Bauer, J. A.; Fackler, Jr., J. P.; Bruce, A. E.; Bruce, M. R. M. J. Chem. Soc. Chem. Commun. 2005, 1575-1577.
Foley, Janet B.; Gay, Stanley E.; Vela, Michael J.; Foxman, Bruce M.; Bruce, Alice E. ; Bruce, Mitchell M. R. M. Eur. J. Inorg. Chem., 2007, 4946-4951.
Chen, J.; Jiang, T.; Wei, G.; Mohamed, A.A.; Homrighausen, C.; Krause Bauer, J.A.; Bruce, A.E.; Bruce, M.R.M JACS, 1999, 121, 9225.
Foley, J.B.; Bruce, A.E.; Bruce, M.R.M. JACS 1995, 117, 9596.





