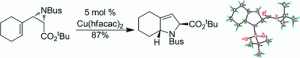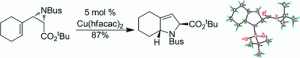Publications
Publications:
17. T. Cheewawisuttichai, M. Brichacek “Development of a multifunctional neoglycoside auxiliary for applications in glycomics research” Org. Biomol. Chem. 2021, 19, 6613-6617.

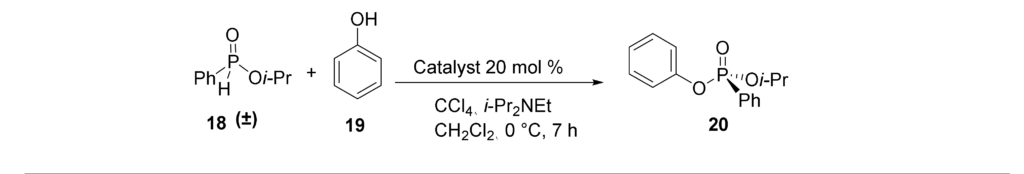
16. A. Numan, M. Brichacek “Asymmetric Synthesis of Stereogenic Phosphorus P(V) Centers Using Chiral Nucleophilic Catalysis” Molecules 2021, 26 (12), 3661.
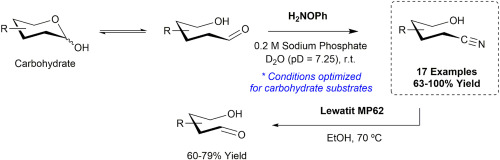
15. T. Cheewawisuttichai, R. D. Hurst, M. Brichacek “Transformation of aldehydes into nitriles in an aqueous medium using O-phenylhydroxylamine as the nitrogen source” Carbohydate Res. 2021, 502, Epub 108282.
14. E. N. Patel, R. B. Arthur, A. D. Nicholas, E. W. Reinheimer, M. A. Omary,M. Brichacek*, H. H. Patterson* “Synthesis, structure and photophysical properties of a 2D network with gold dicyanide donors coordinated to aza[5]helicene viologen acceptors” Dalton Trans. 2019, 48, 10288-10297.
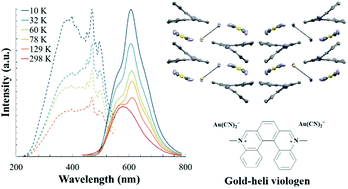 Aza5 helicene viologen
Aza5 helicene viologen
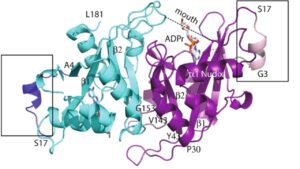
13. P. Thirawatananond, R. L. McPherson, J. Malhi, S. Nathan, M. J. Lambrecht, M. Brichacek, P. J. Hergenrother, A. K. L. Leung, S. B. Gabelli “Structural analyses of NudT16-ADP-ribose complexes direct rational design of mutants with improved processing of poly(ADP-ribosyl)ated proteins.” Sci. Rep. 2019, 9, 5940.
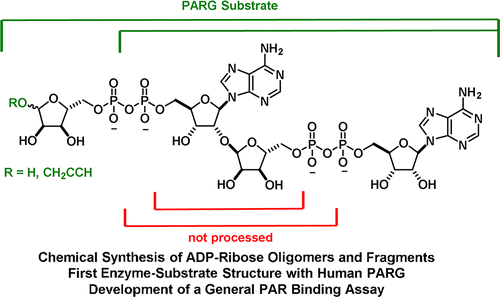
12. M. J. Lambrecht*, M. Brichacek*, E. Barkauskaite, A. Ariza, I. Ahel, P. J. Hergenrother “Synthesis of Dimeric ADP-Ribose and its Structure with Human Poly(ADP-Ribose) Glycohydrolase” J. Am. Chem. Soc. 2015, 137, 3558. *Equal Contribution
11. N. A. McGrath, M. Brichacek “Trifluoroperacetic Acid” Encyclopedia of Reagents for Organic Synthesis 2012, John Wiley & Sons Ltd.
10. F. Li, D. Calabrese, M. Brichacek, I. Lin, J. T. Njardarson “Efficient Synthesis of Thiopyrans Using a Sulfur-Enabled Anionic Cascade” Angew. Chem. Int. Ed. 2012, 51, 1938-1941.
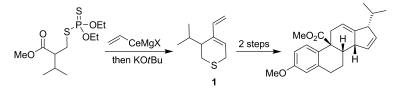
9. M. Brichacek, M. N. Villalobos, A. Plichta, J. T. Njardarson “Stereospecific Ring Expansion of Chiral Vinyl Aziridines” Org. Lett. 2011, 13, 1110-1113.
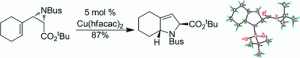
8. N. A. McGrath, J. R. Binner, G. Markopoulos, M. Brichacek, J. T. Njardarson “An Efficient Oxidative Dearomatization-Radical Cyclization Approach to Symmetrically Substituted Bicyclic Guttiferone Natural Products” Chem. Commun. 2010, 47, 209-211.
7. N. A. McGrath, M. Brichacek, J. T. Njardarson “A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives” J. Chem. Ed. 2010, 87, 1348-1349.
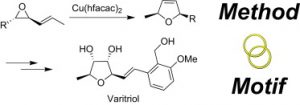
6. M. Brichacek, L. A. Batory, N. A. McGrath, J. T. Njardarson “The Strategic Marriage of Method and Motif. Total Synthesis of Varitriol” Tetrahedron 2010, 66, 4832-4840.
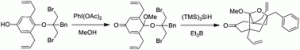
5. M. Brichacek, L. A. Batory, J. T. Njardarson “Stereoselective Ring Expansion of Vinyl Oxiranes. Mechanistic Insights and Natural Product Total Synthesis” Angew. Chem. Int. Ed. 2010, 49, 1648-1651.
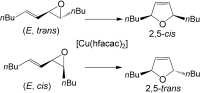
4. M. Brichacek, J. T. Njardarson “Creative approaches towards the synthesis of 2,5-dihydro- furans, thiophenes, and pyrroles. One method does not fit all!” Org. Biomol. Chem. 2009, 7, 1761-1770.
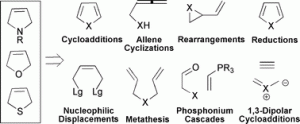
3. N. A. McGrath, C. A. Lee, H. Araki, M. Brichacek, J. T. Njardarson “An Efficient Substrate-Controlled Approach Towards Hypoestoxide, a Member of a Family of Diterpenoid Natural Products with an Inside-Out [9.3.1] Bicyclic Core.” Angew. Chem, Int. Ed. 2008, 47, 9450-9453.
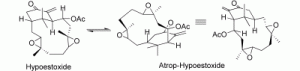
2. M. Brichacek, D. Lee, J. T. Njardarson “Lewis Acid Catalyzed [1,3]-Sigmatropic Rearrangement of Vinyl Aziridines” Org. Lett. 2008, 10, 5023-5026.
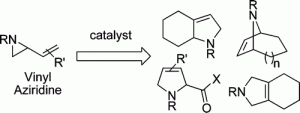
1. M. P. Brichacek, R. M. Carlson “Dihydropyran as a Template for Lactone Synthesis” Synthetic Commun. 2007, 37, 3541-3549.